Published online May 21, 2023. doi: 10.3748/wjg.v29.i19.2888
Peer-review started: February 16, 2023
First decision: March 24, 2023
Revised: April 7, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: May 21, 2023
The main therapeutic options for colorectal cancer are surgical resection and adjuvant chemotherapy in non-metastatic disease. However, the evaluation of the overall adjuvant chemotherapy benefit in patients with a high risk of recurrence is challenging. Radiological images can represent a source of data that can be analyzed by using automated computer-based techniques, working on numerical information coded within Digital Imaging and Communications in Medicine files: This image numerical analysis has been named “radiomics”. Radiomics allows the extraction of quantitative features from radiological images, mainly invisible to the naked eye, that can be further analyzed by artificial intelligence algorithms. Radiomics is expanding in oncology to either understand tumor biology or for the development of imaging biomarkers for diagnosis, staging, and prognosis, prediction of treatment response and diseases monitoring and surveillance. Several efforts have been made to develop radiomics signatures for colorectal cancer patient using computed tomography (CT) images with different aims: The preoperative prediction of lymph node metastasis, detecting BRAF and RAS gene mutations. Moreover, the use of delta-radiomics allows the analysis of variations of the radiomics parameters extracted from CT scans performed at different timepoints. Most published studies concerning radiomics and magnetic resonance imaging (MRI) mainly focused on the response of advanced tumors that under
Core Tip: Stratifying colorectal cancer patients with high-risk disease and the evaluation of the overall chemotherapy benefit are a clinical challenge. Radiomics through radiological images analysis using automated computer-based techniques allows the extraction of quantitative features from radiological images, mainly invisible to the naked eye, that can be further analyzed by artificial intelligence algorithms. Several efforts have been made to develop radiomics signatures for colorectal cancer patient using computed tomography (CT), magnetic resonance imaging, and positron emission tomography/CT, in particular to understand tumor biology, to develop imaging biomarkers for diagnosis, staging, and prognosis, to predict treatment response and to monitor disease.
- Citation: Inchingolo R, Maino C, Cannella R, Vernuccio F, Cortese F, Dezio M, Pisani AR, Giandola T, Gatti M, Giannini V, Ippolito D, Faletti R. Radiomics in colorectal cancer patients. World J Gastroenterol 2023; 29(19): 2888-2904
- URL: https://www.wjgnet.com/1007-9327/full/v29/i19/2888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i19.2888
Colorectal cancer is the fifth-most-common frequent in terms of incidence and mortality, with 1480000 new cases in 2020 worldwide[1]. The TNM staging process itself is widely based on radiological definition of boundaries of primary lesion, nodal and distant metastases. The main therapeutic options for colorectal cancer are surgical resection and adjuvant chemotherapy in non-metastatic patients; however, the evaluation of the overall adjuvant chemotherapy benefit in patients with a high risk of recurrence is a clinical challenge[2]. The decision is based on the TNM staging system[3], which represents the most important parameter: Colorectal cancer patients at stage III are globally recognized as patients who can benefit from chemotherapy, while for those at stage II with other clinical risk factors, the advantages of chemotherapy are still debated[2,4]. In presence of clinical risk factors, the final strategy is often decided by the oncologist or multidisciplinary teams.
Nowadays radiological images can represent a source of data that can be analyzed by using advanced computer-based techniques, working on numerical information coded within the Digital Imaging and Communications in Medicine files[5]: This image numerical analysis has been named “radiomics”[6]. Radiomics might be used as a non-invasive imaging biomarker and be able to provide a quantitative evaluation of medical images, with the chance to shift imaging from a qualitative to a quantitative approach[7,8]. To date, the radiomics approach has been extensively investigated in cancer patients with a specific focus on tumor diagnosis, staging, prognosis prediction, and long-term monitoring[7,9,10]. In this context, radiomics could play a pivotal role in colorectal cancer workup with the expectancy to help clinicians in identifying patients with high-risk disease.
Since its advent in 2012[5], radiomics has been extensively applied in oncology studies and it has been demonstrated as a promising tool that can offer a risk-free and efficient method for diagnosis, classification, and prognosis prediction in oncology[11]. Radiomics, indeed, allows the extraction of quantitative features from radiological images, mainly invisible to the naked eye, that can be further analyzed by machine learning and artificial intelligence (AI) algorithms to produce signatures representing tumor phenotype.
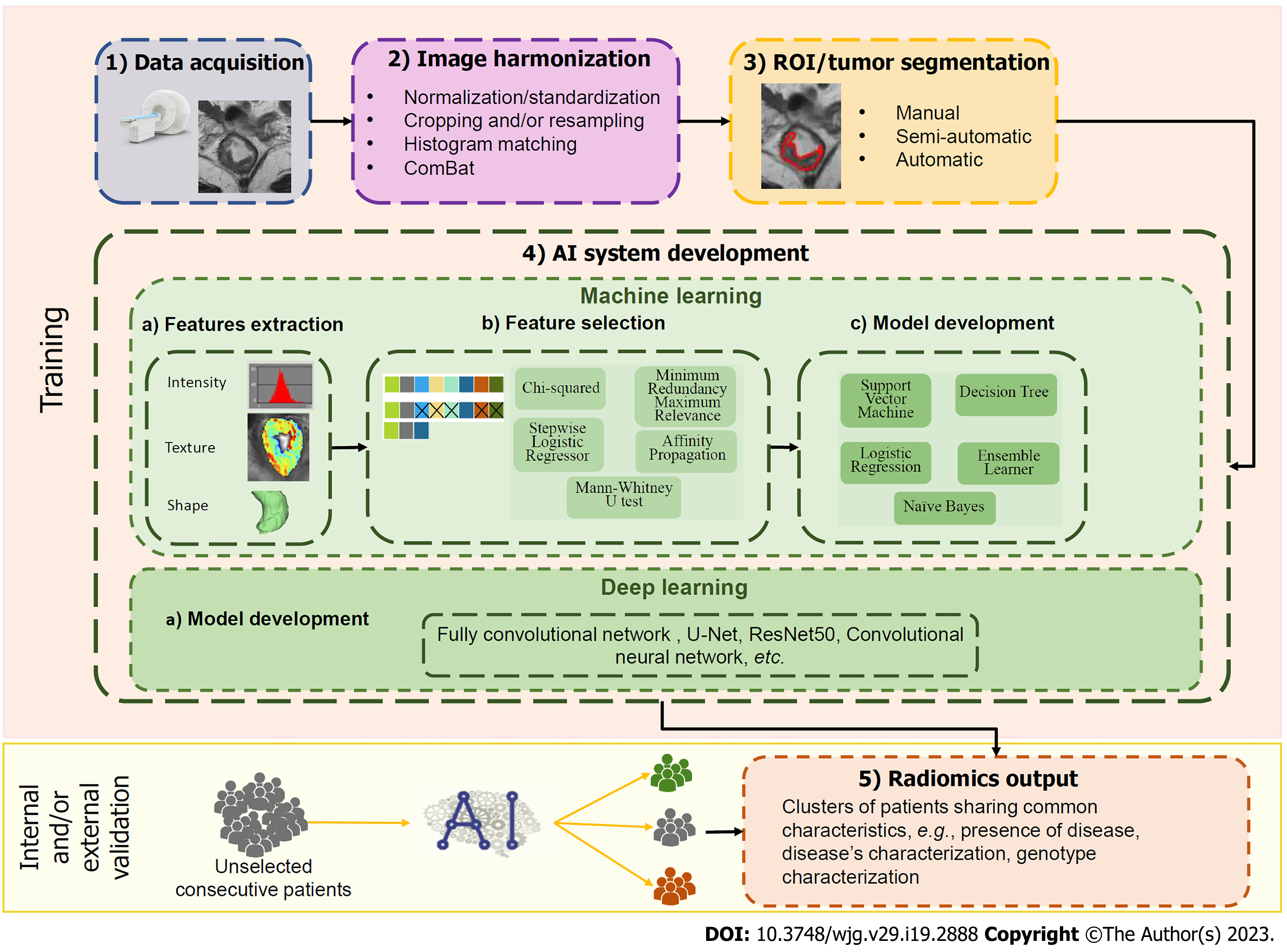
The radiomics pipeline consists of different steps (Figure 1). First, multi-dimensional and multi-institutional data should be collected including high-quality medical images, clinical, and, eventually, molecular data. Once acquired, images should be pre-processed to improve image quality, harmonize raw data, and ensure generalizability across imaging protocols and patient populations, especially if multicentre datasets are acquired. This pre-processing step usually involves image co-registration, image denoising, signal intensity standardization, and/or normalization. Once the image datasets have been pre-processed, tumors should be segmented to extract regions of interest (ROIs) on which subsequent steps will be focused on. This task can be performed either manually, semi- or fully automatically. A big effort in the research field is addressed to the development of AI-based systems to automatically segment lesions and overcomes the most common limitations of manual segmentation. Afterward, from the segmented ROIs a large number of parameters (features) are extracted, including: (1) First-order features, from gray-level intensity histograms and lesion shape; (2) second-order features, related to the spatial relationship between pixels, calculated using different matrices, e.g., gray-level co-occurrence, gray-level run-length, gray-level dependence, gray-level size zone, neighboring gray tone difference; and (3) transform-based features, e.g., Wavelet, Gabor, Laws, Laplacian. However, since the number of extracted features could be much larger than the sample size of patients included in the algorithms’ development, it is vital to reduce the number of features through a step called features selection. This step will strongly reduce the risk of overfitting[12], which occurs when the algorithm overadapts its performances based on data in the training set and consequently loses its generalizability. Besides, features selection will ensue to exclude features that are non-reproducible, redundant, and/or non-relevant for the task, and to reduce the computational cost, while improving the performance of the model[13]. Once the most performing features are selected, the radiomics signature is finally developed by using algorithms for classification, such as logistic regression[14,15], k-nearest neighbour[16], naïve Bayes classifier[17], support vector machines (SVM)[18,19], random forest (RF)[20,21], neural network[22,23] and deep learning[24,25]. In this step, is crucial to divide the image dataset into three subgroups: One for training, used to develop the algorithm, the second for testing, to fine-tune the model, and the last for validation, which aims to evaluate the performance on a different dataset. Training and testing could be performed also using a cross-validation approach, i.e., in which different portions of the dataset are iteratively used to train and test the model. Conversely, the validation should be performed using patients that were never seen during the development of the algorithms. The validation step can be internal, when applied in a similar clinical setting and population to the training set, or preferably external, when applied in multiple clinical settings with varying disease prevalence[12,26].
Thanks to the vast number of images routinely used by radiologists and oncologists in their daily workload, radiomics is substantial in oncology to understand tumor biology or develop imaging biomarkers useful for diagnosis, staging, prediction of treatment response, disease monitoring and surveillance[27].
Understanding tumor biology through radiomics can be feasible because it allows the extraction of quantitative information about spatial and temporal heterogeneity in a non-invasively way and using routinely acquired images. This information can be consequently correlated with tumors’ phenotype that can either reflects distinct traits (e.g., internal necrosis and proliferation at the periphery) or mirrors genomic and molecular traits or be a signature or different outcomes. Moreover, texture features used in radiomics have been also demonstrated useful in reflecting key oncogenomics processes such as tumor angiogenesis[28], hypoxia[29], tumor invasion[30] and tumor proliferation[31].
The second scope of radiomics in the oncology field is to enhance precision medicine through the implementation of diagnostics and prognostic imaging biomarkers in a variety of solid tumors. Biomarkers for detection and diagnosis are those in a more advanced status since there are many studies that demonstrated their usefulness in discriminating between healthy, benign and malignant cancer in different sites[23,32-34]. However, the most promising applications in which radiomics could truly improve clinical practice are related to the prediction of treatment response and disease monitoring. Indeed, knowing, before or during therapy, which patients would respond might help choosing the best management possible. Moreover, after treatment, radiomics biomarkers may suggest more intense post-treatment surveillance due to a high risk of a tumor recurrence for a particular patient. In parallel to radiomics, it is worthwhile underling that a boost for the development of prognostic biomarkers for precision medicine could be provided by integrating radiomics features to additional layers of -omics information, i.e., pathomics (features derived from digital pathological samples), and genomics. The motivation for this multi-omics approach to disease understanding is that the conventional markers discovery which molecularly dissect the disease part by part, if the sum of knowledge of parts will explain the operation of the whole, has mostly failed to understand the causes and cures for complex diseases. On the contrary, recent evidence suggests that patterns discovered from high dimensional, multi-modal data could improve estimation of disease aggressiveness and patient outcomes compared to single modality data.
Several efforts have been made to develop radiomics signatures for colorectal cancer patient using computed tomography (CT) images with different aims (Table 1). Li et al[35] developed and validated a clinical-radiomics nomogram for the preoperative prediction of nodes metastasis. They validated their algorithm on an internal dataset of 308 patients (136 with and 172 without lymph node metastases) and showed that the model which included clinical parameters, radiomics on both tumor and peripheral nodes was the one reaching the highest accuracy in predicting nodes metastases [area under the curve (AUC) = 0.7509; 95%CI: 0.6901-0.8071; accuracy: 73.70%; sensitivity: 60.29%; specificity: 84.30%; positive predictive value (PPV): 75.23%; and negative predictive value (NPV): 72.86% in the internal validation set]. If further validated, also on an external validation set, this model could be used as an individualized preoperative non-invasive tool, assisting in clinical treatment decision making and achieving precision treatment.
| Ref. | Imaging | Main aim | Patients (n) | Main findings |
| Li et al[35], 2020 | CT | Prediction of nodes metastases | 766 | Overall diagnostic values: Sensitivity = 60.3%; specificity = 84.3%; PPV = 75.2%; NPV = 72.9%; AUC = 0.750 |
| Shi et al[16], 2020 | CT | Detect RAS and BRAF phenotypes | 159 | Combined score (semantic features and radiomics) AUC = 0.950; validation cohort AUC = 0.790 |
| Giannini et al[41], 2020 | CT | Predict response to treatment | 38 (141 lesions) | Per-lesion diagnostic values: Sensitivity = 89%; specificity = 85%; PPV = 78%; NPV = 93% |
| Dercle et al[47], 2020 | CT | Tumor response to anti-EGFR therapy | 667 | Sensitivity to therapy: AUCs 0.800 and 0.720 for FOLFIRI and FOLFIRI + cetuximab |
| Dohan et al[48], 2020 | CT | Overall survival | 491 | SPECTRA score > 0.02 has a lower OS; SPECTRA Score at 2 mo has the same prognostic values as RECIST at 6 mo |
| Giannini et al[41], 2020 | CT | Predict response to treatment | 57 (242 lesions) | Per-lesion diagnostic values: Sensitivity = 99%; specificity = 94%; PPV = 95%; NPV = 99%; the radiomic approach can predict R- wrongly classified by RECIST as R+ |
| Taghavi et al[103], 2021 | CT | Prediction of synchronous liver metastases | 91 | The radiomics model outperformed the clinical model: AUC = 0.93 vs 0.64 |
| Rao et al[108], 2014 | CT | Prediction of synchronous liver metastases | 29 | The mean entropy of the liver is significantly higher in metastatic patients (P = 0.02); Liver entropy can help the differential between metastatic and non-metastatic patients (AUC = 0.73-0.78) |
| Li et al[109], 2022 | CT | Prediction of synchronous liver metastases | 323 | A combined clinical-radiomics model has a good AUC (= 0.79) in detecting liver metastases |
| Ng et al[111], 2013 | CT | Prediction of overall survival | 55 | Entropy, uniformity, kurtosis, skewness, and standard deviation of the pixel distribution histogram can predict survival; each parameter can be considered an independent predictor of the overall survival state |
| Mühlberg et al[112], 2021 | CT | Prediction of overall survival | 103 | Tumor burden score can discriminate patients with at least 1-year survival (AUC = 0.70); a machine-learning model better predict survival (AUC = 0.73) |
| Ravanelli et al[116], 2019 | CT | Prediction of response and prognosis after chemotherapy | 43 | Uniformity is lower in responders (P < 0.001); uniformity is independently correlated with radiological response (OR = 20.00), overall survival (RR = 6.94) and progression-free survival (RR = 5.05) |
From another point of view, radiomics has also been proven effective in detecting BRAF and RAS (KRAS and NRAS) gene mutations, that are genomics signatures usually associated with shorter disease-free and overall survival. These mutations are determined through genetic molecular profiling by sampling the tumor, however biopsy carries several drawbacks, including the risk of adverse events, such as bleeding, physical and psychological discomfort[36].
For these reasons, radiomics could potentially be used to non-invasively predict RAS and BRAF mutation status in patients with colorectal cancer and to further guide treatments with surgery or chemotherapy[16,37]. Shi et al[16] validated a combined score that tracks RAS (KRAS and NRAS) and BRAF mutant phenotypes in colorectal cancer from multicentre CT image data AUC of 0.79 on the validation cohort.
Recently, efforts have been made to translate radiomics signatures from a patient level to a lesion level, since it has been demonstrated that heterogeneous response, caused by the onset of new resistant tumor clones in some lesions, is a predictor of poor overall survival[38-40]. Differentiating which colorectal liver metastases (CRLM) responds and which lingers and eventually will progress in the same patient could pave the way to truly personalized treatment. Giannini et al[41] preliminary demonstrated the feasibility of using radiomics features from baseline CT to predict response of treatment after 3 mo. They validated the signature on an independent cohort of patients obtaining encouraging results especially in identifying patients with outlier lesions, i.e., that do not respond in general condition were most lesions respond. In these cases, a target biopsy on non-responder lesions could have revealed a different genetic makeup or, in absence of extrahepatic lesions, suggested the local ablation of outlier metastases. More recently, another breakthrough has been made using the use of delta-radiomics, whose aim is to assess the treatment-induced change of radiomics features over time that could provide information about prognosis[42,43]. These variations can be measured in different ways, for example as the differences between features computed on the same tumour before and after treatment[44,45] or the net-change (i.e., difference of radiomics features after treatment over the value before treatment)[46]. Other than providing additional information about tumour behaviour, delta-radiomics represents a very interesting approach since it could theoretically allow to adapt and modulate the ongoing treatment approach thanks to the predictive power of this technique[42].
Delta-radiomics has been already proven effective in predicting overall survival in patients with metastatic colorectal cancer[47,48]. Dercle et al[47] developed and validated on a multicentre dataset a delta-radiomics associated with tumor sensitivity to anti-EGFR therapy in colorectal cancer patients (AUC = 0.80). Similarly, Dohan et al[48] validated a delta-radiomics signature able to predict overall survival and identify good responders better than RECIST1.1 criteria in patients with metastatic colorectal cancer treated by FOLFIRI and bevacizumab as a first-line treatment. From a per-lesion point of view, Giannini et al[45] validated a delta-radiomics signature able to predict long-term response (i.e., more than 8 mo) of individual CRLM with an accuracy of 86% in the validation dataset. Of note, the delta-radiomics signature was able to reliably predict non-responder liver metastases wrongly classified as responder by lesion RECIST at the first time point. This per-lesion approach could strongly impact treatment, since according to the delta-radiomics signature it would be possible to pinpoint lesions with distinct biological and molecular features, thus enabling studies toward lesion-specific personalized treatment in liver-only metastatic colorectal cancer patients.
The application of radiomics and texture analysis by using magnetic resonance imaging (MRI) images has increased interest in recent years (Table 2), with a specific focus on liver pathology, renal carcinoma, prostate cancer, and, in slight minority of published studies, rectal cancer[49]. As mentioned above, the staging of rectal cancer is mainly based on MRI; however, radiological images analyzed by dedicated software can add important data useful for the best management of patients. In this setting, it is of utmost importance to underline the potentiality of radiomics as a non-invasive biomarker for predicting histopathological data, as demonstrated for different abdominal pathological conditions, related to the liver, pancreas, and colorectal[8]. Even if for many abdominal organs it can be difficult to obtain a useful histological sample, rectal cancer pathological data are easy to collect, considering that colonoscopy or sigmoidoscopy, depending on the location of the lesion, is the reference standard technique[50]. On these bases, most published studies when this search was performed mainly focused on the response of advanced tumors that underwent neoadjuvant therapy.
| Ref. | Imaging | Main aim | Patients (n) | Main findings |
| Horvat et al[52], 2022 | MRI | Response to chemotherapy | 114 | Combined radiological-radiomics model increased agreement (κ = 0.82 vs κ = 0.25) |
| Dinapoli et al[53], 2018 | MRI | Pathological complete response | 221 | Significant covariates, skewness, and entropy can predict pathological complete response, with AUCs = 0.730 and 0.750 for internal and external cohorts |
| Shahzadi et al[50], 2022 | MRI | Response to chemotherapy | 190 | Radiomics combined with the T stage better predict response |
| Liu et al[23], 2021 | MRI | Prediction of nodes metastases | 186 | Clinical-radiomics model improves performance: AUC = 0.827 |
| Chen et al[72], 2022 | MRI | Tumor differentiation and nodes metastases | 37 (487 nodes) | Radiomics features of the primary tumor can predict tumor differentiation: AUC = 0.798 |
| Liu et al[73], 2017 | MRI | Tumor differentiation | 68 | Skewness and entropy are lower in pT1-2 in comparison with pT3-4 (P < 0.05) |
| Yang et al[74], 2019 | MRI | Prediction of T and N stage | 88 | Skewness, kurtosis, and energy are higher in metastatic nodes in comparison with non-metastatic ones (P < 0.001) |
| Ma et al[75], 2019 | MRI | Prediction of nodes metastases and N staging | 152 | SVM has higher diagnostic values for T and N stages (AUC = 0.862) in comparison with MLP and RF |
| Zhu et al[76], 2019 | MRI | Prediction of nodes metastases | 215 | Radiomic model AUC = 0.818 |
| Zhou et al[77], 2020 | MRI | Prediction of nodes metastases | 391 | The combined model predicts nodes metastases: NPV = 93.7%, AUC = 0.818 |
| Shu et al[34], 2019 | MRI | Prediction of synchronous liver metastases | 194 | The Radiomics model combined clinical risk factors and LASSO features and showed a good predictive performance: AUC = 0.921 |
| Liu et al[107], 2020 | MRI | Prediction of synchronous liver metastases | 127 | A radiomic nomogram presents an accuracy of 81.6% in predicting liver metastases (AUC = 0.918) |
| Granata et al[115], 2022 | MRI | Prediction of overall survival | 90 | Second-order features can predict infiltrative tumor growth, tumor budding, and mucinous type; a second-order feature can predict the risk of recurrence with an accuracy of 90% |
| Jalil et al[119], 2017 | MRI | Prediction of prognosis after chemotherapy | 56 | MPP can predict overall survival (HR = 6.9) and disease-free survival (HR = 3.36); texture analysis can predict relapse-free survival on pre- and post-treatment analyses |
Recently, Chen et al[51], in a single-center prospective study, enrolled 137 patients who underwent neoadjuvant chemotherapy. The Authors demonstrated that the traditional clinical model reported an AUC of 67.6% and 70.1% in the training and validation cohort, respectively, quite similar to the selective clinical model (77.5% and 59.6%, respectively). On the other hand, when combining radiomics with clinical data the AUC raised to 94.9% and 84.4% in the training and validation cohort, respectively.
Similarly, Horvat et al[52], by enrolling 114 patients who underwent neoadjuvant chemotherapy, demonstrated that radiomics can help the radiologist determine the pathological complete response. The Authors found that combined clinical and radiomics models increased the agreement compared with radiologist interpretation and can help the less experienced radiologist in increasing diagnostic values, in particular specificity, PPV, and NPV.
Analogously, Dinapoli et al[53] analyzed the radiomics data of 221 patients from three different centers and demonstrated that this tool can help the prediction of pathological complete response before starting neoadjuvant chemotherapy. Moreover, the Authors performed an external validation, to test the obtained results, with good diagnostic values.
Even if few studies are published in the literature, to test the robustness of the radiomics approach in rectal cancer patients, Shahzadi et al[54], demonstrated that only one study can be used for external validation, underlying the overall lack of reproducibility and the need of further standardization before considered it a useful clinical tool. In this setting, future directions should be focused on multicentre studies with standardized MR protocols to validate and test the feasibility of the radiomics approach and its potential usefulness in current everyday clinical practice.
Nodes metastases is the main metastatic site of colorectal cancer and an important cause of post-operative recurrence and death[55]. Nodes status is a key factor in the TNM staging of colorectal cancer and the main determinant of adjuvant chemotherapy[56,57].
Preoperative knowledge of NS can provide valuable information for determining the need for adjuvant therapy and the adequacy of surgical resection, thus aiding in pre-treatment decision making[58].
In clinical practice, CT is the most used preoperative imaging method to detect metastatic lesions and perform tumor staging in patients with colorectal cancer. However, the limitation of CT examination is that it cannot discriminate between benign and malignant nodes[59].
MRI has the highest contrast resolution for soft tissues, allowing the best depiction of neoplastic lesions, their anatomical relationships, the depth of the rectal wall involvement, extramural venous invasion, circumferential resection margins, and the assessment of the N stage. For these reasons, MRI examination is considered the reference standard for locoregional staging and restaging in RC according to the main international guidelines[60-62].
Advances in pattern recognition tools and the increase in data set sizes have facilitated the development of radiomics, which may potentially improve predictive accuracy in oncology[63].
Therefore, in the last decade, several papers have been published, with different imaging techniques, reporting the potential role of radiomics in diagnosis, characterization, and evaluation of the tumor response to treatments[64-67] and nodal assessment[68-70].
MRI can provide multiparameter images different from those obtained by CT, so it is of interest whether there exists an association between NS and multiregional radiomics features of multiparametric MR images in rectal cancer patients[71].
Liu et al[71] aimed to develop and validate a multiregional radiomics prediction model based on MRI and combine it with clinical-semantic data for the individualized preoperative prediction of lymph node metastasis in rectal cancer patients.
Similarly, the study of Chen et al[72] provides two non-invasive and quantitative methods, which respectively predict the tumor differentiation and regional nodes metastases for rectal cancer preoperatively. MRI images of both the primary tumor alongside the lymph nodes and specimens were performed with a node-to-node match and labeling. A prediction model was then successfully developed, which provided AUC values of 84.6% and 73.3% in the training and test cohort, respectively.
Liu et al[73] performed a histogram analysis on the ADC map of the whole tumor and reported that entropy was an independent predictor of nodal involvement. Recently, Yang et al[74] performed the same analysis on T2-weighted imaging of the whole tumor: They found that a lower skewness was an independent risk factor for lymph node metastases.
In a recent retrospective single-center study, radiomics features were extracted from preoperative high-resolution T2-weighted imaging of different histological RC and analyzed using different algorithms. The RF analysis showed a good diagnostic performance for the N-stage with an AUC of 74.6%. The prediction model was able to differentiate N0 from N1-N2 patients with a sensitivity of 79.0% and a specificity of 72.0%[75].
Zhu et al[76] compared the performance of two models based, respectively, on the radiomics signature of the primary tumor and of the lymph nodes, before and after chemoradiotherapy (CRT), for the prediction of nodal involvement in advanced rectal cancer. The authors concluded that the features from the lymph node model perform better than the tumor features for the prediction of nodal involvement[76].
Similarly, Zhou et al[77] evaluated a multi-parametric MRI radiomics model for nodal assessment following CRT by combining the radiomic signature with an experienced radiologist’s visual evaluation: stratified analyses indicated that the combined model could predict lymph node metastasis with a NPV of 100 and 87.8% after treatment[77].
Even if current literature is focusing on the importance and applicability of radiomics in rectal cancer, no significant studies have been published in the field of colorectal cancer, mainly because MR is not still validated as imaging technique for local staging and restaging of this kind of tumor.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is frequently employed in the staging and post-neoadjuvant assessment of patients with rectal cancer. PET/CT provides functional information of the primary tumor, nodes and distant metastases. In patients with colorectal cancer, the combination of texture parameters with the functional information obtained with PET/CT scan can further enhance the predictive power of PET/CT imaging[78]. Current studies mostly focused on the applications of radiomics in PET/CT for the prediction of survival after curative surgical resection and assessment of response following neoadjuvant chemoradiotherapy (Table 3).
| Ref. | Imaging | Main aim | Patients (n) | Main findings |
| Lovinfosse et al[80], 2018 | PET/CT | Progression-free and overall survival | 86 | SUVmean, dissimilarity, and contrast from the neighborhood intensity-difference matrix are independently associated with overall survival |
| Hotta et al[81], 2021 | PET/CT | Progression-free and overall survival | 94 | MTV, TLG, and GLCM entropy are associated with overall survival; SUVmax, MTV, TLG, and GLCM entropy are associated with progression-free survival |
| Bundschuh et al[83], 2014 | PET/CT | Response after neoadjuvant chemotherapy | 27 | COV can assess histopathologic response during (sensitivity 68%, specificity 88%) and after (sensitivity 79%, specificity 88%) therapy |
| Bang et al[84], 2016 | PET/CT | Response after neoadjuvant chemotherapy | 74 | MV is associated with 3-yr disease-free survival; Kurtosis and kurtosis gradient are associated with 3-yr disease-free survival |
| Giannini et al[85], 2019 | PET/CT | Response after neoadjuvant chemotherapy | 52 | Second-order texture features (five from PET and one from MRI) can help distinguish responder and non-responder patients: Sensitivity = 86%; specificity = 83%; AUC = 0.860 |
| Yuan et al[89], 2021 | PET/CT | Response after neoadjuvant chemotherapy | 66 | A radiomics model can predict TRG 0 vs TRG 1-3: Sensitivity = 77.8%, specificity = 89.7%, AUC = 0.858 |
| Schurink et al[86], 2021 | PET/CT | Response after neoadjuvant chemotherapy | 61 | Combined baseline and global tumor features better predict response compared to baseline and local texture (AUC = 0.83 vs 0.79) |
| Shen et al[87], 2020 | PET/CT | Predict pathological complete response | 169 | RF can predict complete response: Sensitivity = 81.8%; specificity = 97.3%; PPV = 81.8%; NPV = 97.3%; accuracy = 95.3% |
| He et al[90], 2021 | PET/CT | Prediction of nodes metastases | 199 | Logist regression and XGBoost can accurately predict nodes metastases with AUC = 0.866 and 0.903, respectively |
| Ma et al[91], 2022 | PET/CT | Prediction of perineural invasion and outcome | 131 | 12 radiomics signatures are associated with peri-neural invasion; a radiomic score can differentiate between perineural positive and negative lesions: AUC = 0.900 |
| Li et al[92], 2021 | PET/CT | Prediction of microsatellite instability | 173 | 2 radiomics features can predict microsatellite instability: Sensitivity = 83.3%; specificity = 76.3%; accuracy = 76.8% |
| Lovinfosse et al[93], 2016 | PET/CT | Prediction of RAS status | 151 | SUVmax, SUV mean, skewness, SUV standard deviation, and SUV coefficient of variation are associated with RAF mutation (all P < 0.001) |
| Chen et al[94], 2019 | PET/CT | Prediction of genetic mutations | 74 | MTV and SUV max are increased in mutated KRAS tumors (all P < 0.001); short-run low gray-level emphasis is associated with p53 mutations (P = 0.001); gray-level zone emphasis is associated with APC mutations (P = 0.006) |
For the prediction of prognosis in patients with colorectal cancer, Kang et al[79] showed that radiomics score from baseline PET scans was significantly associated with progression-free survival. Similarly, Lovinfosse et al[80] and Hotta et al[81] correlated the texture features in PET/CT with both progression-free survival and overall survival. Furthermore, a recent study provided a combined clinical-radiomics model with high predictive performance (C-index of 0.780) for recurrence-free survival in 196 patients with PET/CT[82].
Several studies explored the potential of radiomics for the prediction of response and survival after neoadjuvant chemoradiotherapy[83-89]. In an initial study of 27 patients with rectal cancer treated with neoadjuvant chemoradiotherapy, Bundschuh et al[83] calculated texture parameters (skewness and kurtosis) on PET/CT, which provided a good performance for late response prediction but no significant predictive capability for the assessment of early response. In a retrospective study performed by Bang et al[84], texture parameters extracted from PET images correlated with both tumor regression grading and disease-free survival in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy. Giannini et al[85] combined radiomics features from PET and MRI to predict pathological complete response following neoadjuvant chemoradiotherapy with high accuracy (AUC of 0.86) in patients with rectal cancer. Similarly, Schurink et al[86] combined pretreatment tumor features on PET/CT and MRI to predict response to chemoradiotherapy in rectal cancer, with an AUC of 0.81. Shen et al[87] developed a RF model to predict pathological complete response after neoadjuvant chemoradiotherapy in 169 patients with rectal cancer, which demonstrated a sensitivity of 97.3% and a specificity of 81.8% for the identifications of cancers with complete response. Nevertheless, a study by Karahan Şen et al[88] found no superiority of texture features compared to metabolic tumor volume in predicting response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer.
For the prediction of lymph node metastasis, a retrospective study published by He and colleagues[90] analyzed the radiomics score and five machine-learning models to predict metastatic lymph nodes based on the radiomics features of 199 colorectal cancers, with an AUC of 0.747-0.581 in the test set. Additionally, the performance of PET/CT radiomics features for predicting perineural invasion has been explored in a recent retrospective study[91].
Finally, few other studies explored the correlation between radiomics signature extracted from PET/CT and rectal cancer genotypes, such as microsatellite instability status[92], RAS mutational status[93,94], TP53 and adenomatous polyposis coli mutations[94].
Despite the promising value of radiomics in PET/CT scans of rectal cancer, it should be noted that all current results are based on retrospective single-center studies with heterogeneity on the type of extracted features and analysis. Moreover, several current studies demonstrated insufficient quality according to the radiomics quality score assessment[95].
CRLM develop in about 25% of patients with colorectal carcinoma, being more commonly synchronous (14%-17%) rather than metachronous (8%-15%)[96-99]. CT is most adopted to detect CRLM at preoperative staging due to its higher availability compared to MRI, while MRI is usually used in selected doubtful cases particularly in the challenging scenario of the “too small to characterize” hypoattenuating lesion. In addition, chemotherapy regimens may cause focal or diffuse hepatic changes at imaging that can profoundly alter visualization of hepatic metastases on CT, reducing its diagnostic accuracy and MRI proves to be helpful as problem-solving tool in some cases[100]. Therefore, the overall sensitivity, specificity and accuracy for the diagnosis of CRLM are lower for CT compared to MRI[100-102]. For this reason, at some center, abbreviated gadoxetate disodium MRI protocols are adopted rather than trusting CT only[103,104].
The adoption of radiomics has been proven successful in diagnostic, prognostic, and therapeutic stages[78].
In term of diagnosis of synchronous classical logistic regression models (CLRM), it is relevant to highlight that even with MRI the sensitivity may be lower than 80%, particularly in patients with mucinous adenocarcinoma as primary tumor, prior local treatment in the liver or metastases smaller than 1 cm[102]. Therefore, the first main diagnostic task of radiomics should be the identification of CRLM before they can be seen by radiologist’s naked eye (i.e., detection of synchronous metastases). In a pilot study, Devoto et al[105] proved that radiomics can potentially predict the development of liver metastases on baseline liver CT, by demonstrating a higher heterogeneity of liver texture analysis in patients who developed liver metastases compared to patients who did not develop them. Other authors[34,106] investigated whether radiomics applied to T2-weighted images of the primary tumor on MRI could help in the preoperative prediction of CRLM: Shu et al[34] used a region of interest while Liu et al[107] used a volume of interest and both demonstrated that a radiomics nomogram constructed by combining radiomics and clinical data achieve AUC higher than 90% in the preoperative prediction of CRLM.
The second main diagnostic task of radiomics should be preoperative identification of patients at risk of developing CLRM (i.e., detection of metachronous metastases) based on micro-environmental changes in the apparently normal liver. Taghavi et al[106] and Rao et al[108] designed a prediction model based on liver CT radiomics for the detection of metachronous CRL, with the first study including more patients imaged at three centers and combining radiomics and clinical data achieving AUC of up to 86% in the validation cohort. Other studies tried to achieve the same goal by assessing the primary tumor on CT[109] or MRI[110]. Specifically, Li et al[109] obtained an AUC of 0.72 for the prediction of metachronous CLRM by combining clinical data and volumetric radiomics of the primary tumor on CT. In regard of MRI, a systematic review including 1497 patients estimated a pooled sensitivity and specificity of radiomics applied to rectal MRI of 0.76 and 0.85 respectively in predicting metachronous CLRM, and AUC of the included studies ranging from 0.83 to 0.87[110].
In terms of prognostic information, radiomics of CRLM has emerged as a promising tool to preoperatively predict patient survival at diagnosis and after therapy. Ng et al[111] suggested that tumors demonstrating less texture tumor heterogeneity using radiomic CT analysis may predict poorer survival at diagnosis[111]. Mühlberg et al[112] showed that CT-based geometric distribution and radiomics analysis of whole liver tumor burden from preoperative CT may help for prediction of 1-year survival. Radiomics of CRLM on CT seems also promising for differentiating desmoplastic from replacement histopathological growth patterns[113], and this differentiation may provide an earlier estimate of disease aggressiveness and prognosis as the desmoplastic histopathological growth pattern usually has longer overall survival[114]. As demonstrated by Granata et al[115] contrast MR-based radiomics and machine learning analysis may help in the preoperative prediction of the front of tumor growth (expansive or infiltrative), the tumor budding (absent, low grade or high grade) and tumor recurrence after surgery, all of which may affect patient outcome. Studies looking at survival after chemotherapy in patients with CRLMs obtained similar results regarding the role of texture homogeneity/heterogeneity in the prediction of prognosis[116,117]. As an example, Ravanelli et al[116] demonstrated that lower uniformity of CRLM on CT texture analysis was independently correlated with overall survival and progression free survival in patients treated with bevacizumab, but not in those treated with standard chemotherapy. Other radiomic features such as entropy, kurtosis, and skewness have been investigated so far on CT and MRI, all providing an additional piece of the puzzle and supporting the concept that the addition of texture analysis in the pre-treatment assessment may provide information on prognosis in patients with primary colorectal cancer and CLRM[118,119].
Finally, the correct assessment of response in the treatment of CLRM and the prompt prediction of early response is of utmost important in defining the success or failure of treatment interventions and in the selection of those patients requiring a change of the therapeutic regimen. Chemotherapy for CLRM in the modern era of oxaliplatin- and irinotecan-containing regimens (e.g., FOLFOX, FOLFIRI, CAPOX/FOLFOXIRI, XELOX) has been implemented with the introduction of targeted biologics and immunotherapeutic agents (e.g., bevacizumab, cetuximab, panitumumab, pembrolizumab), thus expanding the proportion of patients eligible for curative-intent surgery, but their use may lead to side effects or complications[120]. Prediction of tumor response before starting chemotherapy would allow to choose the best treatment, avoiding unnecessary adverse effects of the therapy. Radiomics of CLRM has been proven promising for predicting response to different chemotherapy regimens, but the predictive value of radiomics features seems to be treatment dependent[95]. As shown by two systematic reviews[95,121], most studies performed radiomics on CT rather than MRI. In patients treated with FOLFOX or FOLFIRI, low skewness and narrower standard deviation-both suggesting increased tumor homoge
Nowadays much evidence has revealed that not all clinical risk features are equal, not all affect overall survival, and the decision to treat colorectal cancer with adjuvant chemotherapy should be assessed in multidisciplinary approach[123]. In this scenario, radiomics could play a pivotal role in colorectal cancer workup as an additional tool in clinical setting with the expectancy to help clinicians in identifying patients with high-risk disease. In particular, the main fields examined were the preoperative assess
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tari DU, Italy; Yang L, China S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 43574] [Article Influence: 14524.7] [Reference Citation Analysis (47)] |
| 2. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 510] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 3. | Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25:1454-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 4. | Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Lieu C, Mahmoud N, Morris AM, Ruiz-Garcia E, You YN, Meyerhardt JA. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol. 2022;40:892-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 5. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2415] [Cited by in F6Publishing: 3090] [Article Influence: 257.5] [Reference Citation Analysis (2)] |
| 6. | Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2262] [Cited by in F6Publishing: 2842] [Article Influence: 284.2] [Reference Citation Analysis (0)] |
| 7. | Caruso D, Polici M, Zerunian M, Pucciarelli F, Guido G, Polidori T, Landolfi F, Nicolai M, Lucertini E, Tarallo M, Bracci B, Nacci I, Rucci C, Iannicelli E, Laghi A. Radiomics in Oncology, Part 1: Technical Principles and Gastrointestinal Application in CT and MRI. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 8. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1825] [Cited by in F6Publishing: 2729] [Article Influence: 389.9] [Reference Citation Analysis (0)] |
| 9. | Caruso D, Polici M, Zerunian M, Pucciarelli F, Guido G, Polidori T, Landolfi F, Nicolai M, Lucertini E, Tarallo M, Bracci B, Nacci I, Rucci C, Eid M, Iannicelli E, Laghi A. Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Botta F, Raimondi S, Rinaldi L, Bellerba F, Corso F, Bagnardi V, Origgi D, Minelli R, Pitoni G, Petrella F, Spaggiari L, Morganti AG, Del Grande F, Bellomi M, Rizzo S. Association of a CT-Based Clinical and Radiomics Score of Non-Small Cell Lung Cancer (NSCLC) with Lymph Node Status and Overall Survival. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Fan G, Qin J, Liu H, Liao X. Commentary: Radiomics in oncology: A 10-year bibliometric analysis. Front Oncol. 2022;12:891056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 12. | van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging. 2020;11:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 485] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 13. | Defeudis A, De Mattia C, Rizzetto F, Calderoni F, Mazzetti S, Torresin A, Vanzulli A, Regge D, Giannini V. Standardization of CT radiomics features for multi-center analysis: impact of software settings and parameters. Phys Med Biol. 2020;65:195012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Pan S, Ding Z, Zhang L, Ruan M, Shan Y, Deng M, Pang P, Shen Q. A Nomogram Combined Radiomic and Semantic Features as Imaging Biomarker for Classification of Ovarian Cystadenomas. Front Oncol. 2020;10:895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Qi Y, Zhang S, Wei J, Zhang G, Lei J, Yan W, Xiao Y, Yan S, Xue H, Feng F, Sun H, Tian J, Jin Z. Multiparametric MRI-Based Radiomics for Prostate Cancer Screening With PSA in 4-10 ng/mL to Reduce Unnecessary Biopsies. J Magn Reson Imaging. 2020;51:1890-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Shi R, Chen W, Yang B, Qu J, Cheng Y, Zhu Z, Gao Y, Wang Q, Liu Y, Li Z, Qu X. Prediction of KRAS, NRAS and BRAF status in colorectal cancer patients with liver metastasis using a deep artificial neural network based on radiomics and semantic features. Am J Cancer Res. 2020;10:4513-4526. [PubMed] [Cited in This Article: ] |
| 17. | Frøkjær JB, Lisitskaya MV, Jørgensen AS, Østergaard LR, Hansen TM, Drewes AM, Olesen SS. Pancreatic magnetic resonance imaging texture analysis in chronic pancreatitis: a feasibility and validation study. Abdom Radiol (NY). 2020;45:1497-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Petkovska I, Tixier F, Ortiz EJ, Golia Pernicka JS, Paroder V, Bates DD, Horvat N, Fuqua J, Schilsky J, Gollub MJ, Garcia-Aguilar J, Veeraraghavan H. Clinical utility of radiomics at baseline rectal MRI to predict complete response of rectal cancer after chemoradiation therapy. Abdom Radiol (NY). 2020;45:3608-3617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Wang JC, Fu R, Tao XW, Mao YF, Wang F, Zhang ZC, Yu WW, Chen J, He J, Sun BC. A radiomics-based model on non-contrast CT for predicting cirrhosis: make the most of image data. Biomark Res. 2020;8:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Park S, Chu LC, Hruban RH, Vogelstein B, Kinzler KW, Yuille AL, Fouladi DF, Shayesteh S, Ghandili S, Wolfgang CL, Burkhart R, He J, Fishman EK, Kawamoto S. Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging. 2020;101:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Gu D, Hu Y, Ding H, Wei J, Chen K, Liu H, Zeng M, Tian J. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur Radiol. 2019;29:6880-6890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 22. | Bagher-Ebadian H, Janic B, Liu C, Pantelic M, Hearshen D, Elshaikh M, Movsas B, Chetty IJ, Wen N. Detection of Dominant Intra-prostatic Lesions in Patients With Prostate Cancer Using an Artificial Neural Network and MR Multi-modal Radiomics Analysis. Front Oncol. 2019;9:1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Fan H, Dong D, Liu P, He B, Meng L, Chen J, Chen C, Lang J, Tian J. Computed tomography-based radiomic model at node level for the prediction of normal-sized lymph node metastasis in cervical cancer. Transl Oncol. 2021;14:101113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Panic J, Defeudis A, Mazzetti S, Rosati S, Giannetto G, Vassallo L, Regge D, Balestra G, Giannini V. A Convolutional Neural Network based system for Colorectal cancer segmentation on MRI images. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1675-1678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Barra D, Nicoletti G, Defeudis A, Mazzetti S, Panic J, Gatti M, Faletti R, Russo F, Regge D, Giannini V. Deep learning model for automatic prostate segmentation on bicentric T2w images with and without endorectal coil. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:3370-3373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Riley RD, Snell KIE, Ensor J, Burke DL, Harrell FE Jr, Moons KGM, Collins GS. Minimum sample size for developing a multivariable prediction model: Part I - Continuous outcomes. Stat Med. 2019;38:1262-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 27. | Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S, Schernberg A, Paragios N, Deutsch E, Ferté C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol. 2017;28:1191-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 28. | Ganeshan B, Abaleke S, Young RC, Chatwin CR, Miles KA. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging. 2010;10:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Gevaert O, Xu J, Hoang CD, Leung AN, Xu Y, Quon A, Rubin DL, Napel S, Plevritis SK. Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data--methods and preliminary results. Radiology. 2012;264:387-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 30. | Zhu Y, Li H, Guo W, Drukker K, Lan L, Giger ML, Ji Y. Deciphering Genomic Underpinnings of Quantitative MRI-based Radiomic Phenotypes of Invasive Breast Carcinoma. Sci Rep. 2015;5:17787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY, Kuo MD. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007;25:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 399] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 32. | Reginelli A, Capasso R, Petrillo M, Rossi C, Faella P, Grassi R, Belfiore MP, Rossi G, Muto M, Muto P, Fiorello A, Serra N, Nizzoli R, De Filippo M, Cappabianca S, Carrafiello G, Brunese L, Rotondo A. Looking for Lepidic Component inside Invasive Adenocarcinomas Appearing as CT Solid Solitary Pulmonary Nodules (SPNs): CT Morpho-Densitometric Features and 18-FDG PET Findings. Biomed Res Int. 2019;2019:7683648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Chiappa V, Bogani G, Interlenghi M, Salvatore C, Bertolina F, Sarpietro G, Signorelli M, Castiglioni I, Raspagliesi F. The Adoption of Radiomics and machine learning improves the diagnostic processes of women with Ovarian MAsses (the AROMA pilot study). J Ultrasound. 2021;24:429-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Shu Z, Fang S, Ding Z, Mao D, Cai R, Chen Y, Pang P, Gong X. MRI-based Radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci Rep. 2019;9:3374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Li M, Zhang J, Dan Y, Yao Y, Dai W, Cai G, Yang G, Tong T. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med. 2020;18:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 36. | Purnell SD, Bloom JB, Valera V, Wood BJ, Turkbey B, Pinto PA. Targeted biopsy: benefits and limitations. Curr Opin Urol. 2018;28:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Negreros-Osuna AA, Parakh A, Corcoran RB, Pourvaziri A, Kambadakone A, Ryan DP, Sahani DV. Radiomics Texture Features in Advanced Colorectal Cancer: Correlation with BRAF Mutation and 5-year Overall Survival. Radiol Imaging Cancer. 2020;2:e190084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | van Kessel CS, Samim M, Koopman M, van den Bosch MA, Borel Rinkes IH, Punt CJ, van Hillegersberg R. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer. 2013;49:2486-2493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, Mussolin B, Kwak EL, Buscarino M, Lazzari L, Valtorta E, Truini M, Jessop NA, Robinson HE, Hong TS, Mino-Kenudson M, Di Nicolantonio F, Thabet A, Sartore-Bianchi A, Siena S, Iafrate AJ, Bardelli A, Corcoran RB. Tumor Heterogeneity and Lesion-Specific Response to Targeted Therapy in Colorectal Cancer. Cancer Discov. 2016;6:147-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 40. | Piotrowska Z, Niederst MJ, Karlovich CA, Wakelee HA, Neal JW, Mino-Kenudson M, Fulton L, Hata AN, Lockerman EL, Kalsy A, Digumarthy S, Muzikansky A, Raponi M, Garcia AR, Mulvey HE, Parks MK, DiCecca RH, Dias-Santagata D, Iafrate AJ, Shaw AT, Allen AR, Engelman JA, Sequist LV. Heterogeneity Underlies the Emergence of EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive Cancers with a Third-Generation EGFR Inhibitor. Cancer Discov. 2015;5:713-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 41. | Giannini V, Rosati S, Defeudis A, Balestra G, Vassallo L, Cappello G, Mazzetti S, De Mattia C, Rizzetto F, Torresin A, Sartore-Bianchi A, Siena S, Vanzulli A, Leone F, Zagonel V, Marsoni S, Regge D. Radiomics predicts response of individual HER2-amplified colorectal cancer liver metastases in patients treated with HER2-targeted therapy. Int J Cancer. 2020;147:3215-3223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Nardone V, Reginelli A, Grassi R, Boldrini L, Vacca G, D'Ippolito E, Annunziata S, Farchione A, Belfiore MP, Desideri I, Cappabianca S. Delta radiomics: a systematic review. Radiol Med. 2021;126:1571-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 43. | Nasief H, Zheng C, Schott D, Hall W, Tsai S, Erickson B, Allen Li X. A machine learning based delta-radiomics process for early prediction of treatment response of pancreatic cancer. NPJ Precis Oncol. 2019;3:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 44. | Jeon SH, Song C, Chie EK, Kim B, Kim YH, Chang W, Lee YJ, Chung JH, Chung JB, Lee KW, Kang SB, Kim JS. Delta-radiomics signature predicts treatment outcomes after preoperative chemoradiotherapy and surgery in rectal cancer. Radiat Oncol. 2019;14:43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Giannini V, Pusceddu L, Defeudis A, Nicoletti G, Cappello G, Mazzetti S, Sartore-Bianchi A, Siena S, Vanzulli A, Rizzetto F, Fenocchio E, Lazzari L, Bardelli A, Marsoni S, Regge D. Delta-Radiomics Predicts Response to First-Line Oxaliplatin-Based Chemotherapy in Colorectal Cancer Patients with Liver Metastases. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Fave X, Zhang L, Yang J, Mackin D, Balter P, Gomez D, Followill D, Jones AK, Stingo F, Liao Z, Mohan R, Court L. Delta-radiomics features for the prediction of patient outcomes in non-small cell lung cancer. Sci Rep. 2017;7:588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 47. | Dercle L, Lu L, Schwartz LH, Qian M, Tejpar S, Eggleton P, Zhao B, Piessevaux H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J Natl Cancer Inst. 2020;112:902-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 48. | Dohan A, Gallix B, Guiu B, Le Malicot K, Reinhold C, Soyer P, Bennouna J, Ghiringhelli F, Barbier E, Boige V, Taieb J, Bouché O, François E, Phelip JM, Borel C, Faroux R, Seitz JF, Jacquot S, Ben Abdelghani M, Khemissa-Akouz F, Genet D, Jouve JL, Rinaldi Y, Desseigne F, Texereau P, Suc E, Lepage C, Aparicio T, Hoeffel C; PRODIGE 9 Investigators and PRODIGE 20 Investigators. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2020;69:531-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 49. | Thomas JV, Abou Elkassem AM, Ganeshan B, Smith AD. MR Imaging Texture Analysis in the Abdomen and Pelvis. Magn Reson Imaging Clin N Am. 2020;28:447-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1112] [Cited by in F6Publishing: 1148] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 51. | Chen BY, Xie H, Li Y, Jiang XH, Xiong L, Tang XF, Lin XF, Li L, Cai PQ. MRI-Based Radiomics Features to Predict Treatment Response to Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer: A Single Center, Prospective Study. Front Oncol. 2022;12:801743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (1)] |
| 52. | Horvat N, Veeraraghavan H, Nahas CSR, Bates DDB, Ferreira FR, Zheng J, Capanu M, Fuqua JL 3rd, Fernandes MC, Sosa RE, Jayaprakasam VS, Cerri GG, Nahas SC, Petkovska I. Combined artificial intelligence and radiologist model for predicting rectal cancer treatment response from magnetic resonance imaging: an external validation study. Abdom Radiol (NY). 2022;47:2770-2782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Dinapoli N, Barbaro B, Gatta R, Chiloiro G, Casà C, Masciocchi C, Damiani A, Boldrini L, Gambacorta MA, Dezio M, Mattiucci GC, Balducci M, van Soest J, Dekker A, Lambin P, Fiorino C, Sini C, De Cobelli F, Di Muzio N, Gumina C, Passoni P, Manfredi R, Valentini V. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int J Radiat Oncol Biol Phys. 2018;102:765-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 54. | Shahzadi I, Zwanenburg A, Lattermann A, Linge A, Baldus C, Peeken JC, Combs SE, Diefenhardt M, Rödel C, Kirste S, Grosu AL, Baumann M, Krause M, Troost EGC, Löck S. Analysis of MRI and CT-based radiomics features for personalized treatment in locally advanced rectal cancer and external validation of published radiomics models. Sci Rep. 2022;12:10192. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 55. | Xue H, Du X, Xiao C, Yan Y, Zou Z, Xu Y. [Predictive value of lymph node ratio for postoperative distant metastasis of stage III colorectal cancer]. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:458-462. [PubMed] [Cited in This Article: ] |
| 56. | Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1-34. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Sabbagh C, Mauvais F, Cosse C, Rebibo L, Joly JP, Dromer D, Aubert C, Carton S, Dron B, Dadamessi I, Maes B, Perrier G, Manaouil D, Fontaine JF, Gozy M, Panis X, Foncelle PH, de Fresnoy H, Leroux F, Vaneslander P, Ghighi C, Regimbeau JM; APCD-LNR-2011 study group. A lymph node ratio of 10% is predictive of survival in stage III colon cancer: a French regional study. Int Surg. 2014;99:344-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, Ma ZL, Liu ZY. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol. 2016;34:2157-2164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 1167] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 59. | Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A'Hern R, Brown G. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65:708-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 268] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 61. | Nicholls RJ, Zinicola R, Haboubi N. Extramural spread of rectal cancer and the AJCC Cancer Staging Manual 8th edition, 2017. Ann Oncol. 2019;30:1394-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 932] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 63. | Langs G, Röhrich S, Hofmanninger J, Prayer F, Pan J, Herold C, Prosch H. Machine learning: from radiomics to discovery and routine. Radiologe. 2018;58:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X, Sun K, Li L, Li B, Wang M, Tian J. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019;9:1303-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 447] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 65. | Pinto Dos Santos D, Dietzel M, Baessler B. A decade of radiomics research: are images really data or just patterns in the noise? Eur Radiol. 2021;31:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 66. | Kirienko M, Ninatti G, Cozzi L, Voulaz E, Gennaro N, Barajon I, Ricci F, Carlo-Stella C, Zucali P, Sollini M, Balzarini L, Chiti A. Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms vs lymphomas. Radiol Med. 2020;125:951-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Karmazanovsky G, Gruzdev I, Tikhonova V, Kondratyev E, Revishvili A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol Med. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Calabrese A, Santucci D, Landi R, Beomonte Zobel B, Faiella E, de Felice C. Radiomics MRI for lymph node status prediction in breast cancer patients: the state of art. J Cancer Res Clin Oncol. 2021;147:1587-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Benedetti G, Mori M, Panzeri MM, Barbera M, Palumbo D, Sini C, Muffatti F, Andreasi V, Steidler S, Doglioni C, Partelli S, Manzoni M, Falconi M, Fiorino C, De Cobelli F. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol Med. 2021;126:745-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 70. | Qin H, Que Q, Lin P, Li X, Wang XR, He Y, Chen JQ, Yang H. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): a comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol Med. 2021;126:1312-1327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Liu X, Yang Q, Zhang C, Sun J, He K, Xie Y, Zhang Y, Fu Y, Zhang H. Multiregional-Based Magnetic Resonance Imaging Radiomics Combined With Clinical Data Improves Efficacy in Predicting Lymph Node Metastasis of Rectal Cancer. Front Oncol. 2020;10:585767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 72. | Chen P, Wen D, Huang L, Ding J, Yang W, Sun J, Yang L, Zhou Z. Magnetic Resonance Imaging Radiomics-Based Model to Identify the Pathological Features and Lymph Node Metastasis in Rectal Cancer. May 3, 2022. [cited 1 March 2023]. Available from: https://www.medrxiv.org/content/10.1101/2022.05.02.22274247v1. [Cited in This Article: ] |
| 73. | Liu L, Liu Y, Xu L, Li Z, Lv H, Dong N, Li W, Yang Z, Wang Z, Jin E. Application of texture analysis based on apparent diffusion coefficient maps in discriminating different stages of rectal cancer. J Magn Reson Imaging. 2017;45:1798-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 74. | Yang L, Liu D, Fang X, Wang Z, Xing Y, Ma L, Wu B. Rectal cancer: can T2WI histogram of the primary tumor help predict the existence of lymph node metastasis? Eur Radiol. 2019;29:6469-6476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | Ma X, Shen F, Jia Y, Xia Y, Li Q, Lu J. MRI-based radiomics of rectal cancer: preoperative assessment of the pathological features. BMC Med Imaging. 2019;19:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Zhu H, Zhang X, Li X, Shi Y, Zhu H, Sun Y. Prediction of pathological nodal stage of locally advanced rectal cancer by collective features of multiple lymph nodes in magnetic resonance images before and after neoadjuvant chemoradiotherapy. Chin J Cancer Res. 2019;31:984-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Zhou X, Yi Y, Liu Z, Zhou Z, Lai B, Sun K, Li L, Huang L, Feng Y, Cao W, Tian J. Radiomics-Based Preoperative Prediction of Lymph Node Status Following Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Front Oncol. 2020;10:604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Vernuccio F, Cannella R, Comelli A, Salvaggio G, Lagalla R, Midiri M. [Radiomics and artificial intelligence: new frontiers in medicine]. Recenti Prog Med. 2020;111:130-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 79. | Kang J, Lee JH, Lee HS, Cho ES, Park EJ, Baik SH, Lee KY, Park C, Yeu Y, Clemenceau JR, Park S, Xu H, Hong C, Hwang TH. Radiomics Features of (18)F-Fluorodeoxyglucose Positron-Emission Tomography as a Novel Prognostic Signature in Colorectal Cancer. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Lovinfosse P, Polus M, Van Daele D, Martinive P, Daenen F, Hatt M, Visvikis D, Koopmansch B, Lambert F, Coimbra C, Seidel L, Albert A, Delvenne P, Hustinx R. FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur J Nucl Med Mol Imaging. 2018;45:365-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 81. | Hotta M, Minamimoto R, Gohda Y, Miwa K, Otani K, Kiyomatsu T, Yano H. Prognostic value of (18)F-FDG PET/CT with texture analysis in patients with rectal cancer treated by surgery. Ann Nucl Med. 2021;35:843-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Lv L, Xin B, Hao Y, Yang Z, Xu J, Wang L, Wang X, Song S, Guo X. Radiomic analysis for predicting prognosis of colorectal cancer from preoperative (18)F-FDG PET/CT. J Transl Med. 2022;20:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 83. | Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, Rosenberg R, Becker K, Astner ST, Henninger M, Herrmann K, Ziegler SI, Schwaiger M, Essler M. Textural Parameters of Tumor Heterogeneity in ¹F-FDG PET/CT for Therapy Response Assessment and Prognosis in Patients with Locally Advanced Rectal Cancer. J Nucl Med. 2014;55:891-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 84. | Bang JI, Ha S, Kang SB, Lee KW, Lee HS, Kim JS, Oh HK, Lee HY, Kim SE. Prediction of neoadjuvant radiation chemotherapy response and survival using pretreatment [(18)F]FDG PET/CT scans in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging. 2016;43:422-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Giannini V, Mazzetti S, Bertotto I, Chiarenza C, Cauda S, Delmastro E, Bracco C, Di Dia A, Leone F, Medico E, Pisacane A, Ribero D, Stasi M, Regge D. Predicting locally advanced rectal cancer response to neoadjuvant therapy with (18)F-FDG PET and MRI radiomics features. Eur J Nucl Med Mol Imaging. 2019;46:878-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 86. | Schurink NW, van Kranen SR, Berbee M, van Elmpt W, Bakers FCH, Roberti S, van Griethuysen JJM, Min LA, Lahaye MJ, Maas M, Beets GL, Beets-Tan RGH, Lambregts DMJ. Studying local tumour heterogeneity on MRI and FDG-PET/CT to predict response to neoadjuvant chemoradiotherapy in rectal cancer. Eur Radiol. 2021;31:7031-7038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Shen WC, Chen SW, Wu KC, Lee PY, Feng CL, Hsieh TC, Yen KY, Kao CH. Predicting pathological complete response in rectal cancer after chemoradiotherapy with a random forest using (18)F-fluorodeoxyglucose positron emission tomography and computed tomography radiomics. Ann Transl Med. 2020;8:207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Karahan Şen NP, Aksu A, Kaya GÇ. Value of volumetric and textural analysis in predicting the treatment response in patients with locally advanced rectal cancer. Ann Nucl Med. 2020;34:960-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Yuan Z, Frazer M, Rishi A, Latifi K, Tomaszewski MR, Moros EG, Feygelman V, Felder S, Sanchez J, Dessureault S, Imanirad I, Kim RD, Harrison LB, Hoffe SE, Zhang GG, Frakes JM. Pretreatment CT and PET radiomics predicting rectal cancer patients in response to neoadjuvant chemoradiotherapy. Rep Pract Oncol Radiother. 2021;26:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | He J, Wang Q, Zhang Y, Wu H, Zhou Y, Zhao S. Preoperative prediction of regional lymph node metastasis of colorectal cancer based on (18)F-FDG PET/CT and machine learning. Ann Nucl Med. 2021;35:617-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Ma J, Guo D, Miao W, Wang Y, Yan L, Wu F, Zhang C, Zhang R, Zuo P, Yang G, Wang Z. The value of (18)F-FDG PET/CT-based radiomics in predicting perineural invasion and outcome in non-metastatic colorectal cancer. Abdom Radiol (NY). 2022;47:1244-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |
| 92. | Li J, Yang Z, Xin B, Hao Y, Wang L, Song S, Xu J, Wang X. Quantitative Prediction of Microsatellite Instability in Colorectal Cancer With Preoperative PET/CT-Based Radiomics. Front Oncol. 2021;11:702055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Lovinfosse P, Koopmansch B, Lambert F, Jodogne S, Kustermans G, Hatt M, Visvikis D, Seidel L, Polus M, Albert A, Delvenne P, Hustinx R. (18)F-FDG PET/CT imaging in rectal cancer: relationship with the RAS mutational status. Br J Radiol. 2016;89:20160212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 94. | Chen SW, Shen WC, Chen WT, Hsieh TC, Yen KY, Chang JG, Kao CH. Metabolic Imaging Phenotype Using Radiomics of [(18)F]FDG PET/CT Associated with Genetic Alterations of Colorectal Cancer. Mol Imaging Biol. 2019;21:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Staal FCR, van der Reijd DJ, Taghavi M, Lambregts DMJ, Beets-Tan RGH, Maas M. Radiomics for the Prediction of Treatment Outcome and Survival in Patients With Colorectal Cancer: A Systematic Review. Clin Colorectal Cancer. 2021;20:52-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 96. | Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 97. | Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 98. | Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 826] [Cited by in F6Publishing: 921] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 99. | Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 645] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 100. | Vernuccio F, Dioguardi Burgio M, Barbiera F, Cusmà S, Badalamenti G, Midiri M, Vilgrain V, Brancatelli G. CT and MR imaging of chemotherapy-induced hepatopathy. Abdom Radiol (NY). 2019;44:3312-3324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Schulz A, Viktil E, Godt JC, Johansen CK, Dormagen JB, Holtedahl JE, Labori KJ, Bach-Gansmo T, Kløw NE. Diagnostic performance of CT, MRI and PET/CT in patients with suspected colorectal liver metastases: the superiority of MRI. Acta Radiol. 2016;57:1040-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Sivesgaard K, Larsen LP, Sørensen M, Kramer S, Schlander S, Amanavicius N, Bharadwaz A, Tønner Nielsen D, Viborg Mortensen F, Morre Pedersen E. Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol. 2018;28:4735-4747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Vernuccio F, Cannella R, Bartolotta TV, Galia M, Tang A, Brancatelli G. Advances in liver US, CT, and MRI: moving toward the future. Eur Radiol Exp. 2021;5:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Canellas R, Rosenkrantz AB, Taouli B, Sala E, Saini S, Pedrosa I, Wang ZJ, Sahani DV. Abbreviated MRI Protocols for the Abdomen. Radiographics. 2019;39:744-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Devoto L, Ganeshan B, Keller D, Groves A, Endozo R, Arulampalam T, Chand M. Using texture analysis in the development of a potential radiomic signature for early identification of hepatic metastasis in colorectal cancer. Eur J Radiol Open. 2022;9:100415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Taghavi M, Trebeschi S, Simões R, Meek DB, Beckers RCJ, Lambregts DMJ, Verhoef C, Houwers JB, van der Heide UA, Beets-Tan RGH, Maas M. Machine learning-based analysis of CT radiomics model for prediction of colorectal metachronous liver metastases. Abdom Radiol (NY). 2021;46:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 107. | Liu M, Ma X, Shen F, Xia Y, Jia Y, Lu J. MRI-based radiomics nomogram to predict synchronous liver metastasis in primary rectal cancer patients. Cancer Med. 2020;9:5155-5163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 108. | Rao SX, Lambregts DM, Schnerr RS, van Ommen W, van Nijnatten TJ, Martens MH, Heijnen LA, Backes WH, Verhoef C, Zeng MS, Beets GL, Beets-Tan RG. Whole-liver CT texture analysis in colorectal cancer: Does the presence of liver metastases affect the texture of the remaining liver? United European Gastroenterol J. 2014;2:530-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | Li Y, Gong J, Shen X, Li M, Zhang H, Feng F, Tong T. Assessment of Primary Colorectal Cancer CT Radiomics to Predict Metachronous Liver Metastasis. Front Oncol. 2022;12:861892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 110. | Davey MS, Davey MG, Ryan ÉJ, Hogan AM, Kerin MJ, Joyce M. The use of radiomic analysis of magnetic resonance imaging in predicting distant metastases of rectal carcinoma following surgical resection: A systematic review and meta-analysis. Colorectal Dis. 2021;23:3065-3072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology. 2013;266:177-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 112. | Mühlberg A, Holch JW, Heinemann V, Huber T, Moltz J, Maurus S, Jäger N, Liu L, Froelich MF, Katzmann A, Gresser E, Taubmann O, Sühling M, Nörenberg D. The relevance of CT-based geometric and radiomics analysis of whole liver tumor burden to predict survival of patients with metastatic colorectal cancer. Eur Radiol. 2021;31:834-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Starmans MPA, Buisman FE, Renckens M, Willemssen FEJA, van der Voort SR, Groot Koerkamp B, Grünhagen DJ, Niessen WJ, Vermeulen PB, Verhoef C, Visser JJ, Klein S. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis. 2021;38:483-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 114. | Galjart B, Nierop PMH, van der Stok EP, van den Braak RRJC, Höppener DJ, Daelemans S, Dirix LY, Verhoef C, Vermeulen PB, Grünhagen DJ. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22:355-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 115. | Granata V, Fusco R, De Muzio F, Cutolo C, Setola SV, Dell' Aversana F, Ottaiano A, Avallone A, Nasti G, Grassi F, Pilone V, Miele V, Brunese L, Izzo F, Petrillo A. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 116. | Ravanelli M, Agazzi GM, Tononcelli E, Roca E, Cabassa P, Baiocchi G, Berruti A, Maroldi R, Farina D. Texture features of colorectal liver metastases on pretreatment contrast-enhanced CT may predict response and prognosis in patients treated with bevacizumab-containing chemotherapy: a pilot study including comparison with standard chemotherapy. Radiol Med. 2019;124:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Andersen IR, Thorup K, Andersen MB, Olesen R, Mortensen FV, Nielsen DT, Rasmussen F. Texture in the monitoring of regorafenib therapy in patients with colorectal liver metastases. Acta Radiol. 2019;60:1084-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Fiz F, Viganò L, Gennaro N, Costa G, La Bella L, Boichuk A, Cavinato L, Sollini M, Politi LS, Chiti A, Torzilli G. Radiomics of Liver Metastases: A Systematic Review. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Jalil O, Afaq A, Ganeshan B, Patel UB, Boone D, Endozo R, Groves A, Sizer B, Arulampalam T. Magnetic resonance based texture parameters as potential imaging biomarkers for predicting long-term survival in locally advanced rectal cancer treated by chemoradiotherapy. Colorectal Dis. 2017;19:349-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 120. | Albano D, Benenati M, Bruno A, Bruno F, Calandri M, Caruso D, Cozzi D, De Robertis R, Gentili F, Grazzini I, Micci G, Palmisano A, Pessina C, Scalise P, Vernuccio F, Barile A, Miele V, Grassi R, Messina C; Young SIRM Working Group. Imaging side effects and complications of chemotherapy and radiation therapy: a pictorial review from head to toe. Insights Imaging. 2021;12:76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 121. | Wesdorp NJ, van Goor VJ, Kemna R, Jansma EP, van Waesberghe JHTM, Swijnenburg RJ, Punt CJA, Huiskens J, Kazemier G. Advanced image analytics predicting clinical outcomes in patients with colorectal liver metastases: A systematic review of the literature. Surg Oncol. 2021;38:101578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Ahn SJ, Kim JH, Park SJ, Han JK. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis. Eur J Radiol. 2016;85:1867-1874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 123. | Babcock BD, Aljehani MA, Jabo B, Choi AH, Morgan JW, Selleck MJ, Luca F, Raskin E, Reeves ME, Garberoglio CA, Lum SS, Senthil M. High-Risk Stage II Colon Cancer: Not All Risks Are Created Equal. Ann Surg Oncol. 2018;25:1980-1985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 124. | Golia Pernicka JS, Gagniere J, Chakraborty J, Yamashita R, Nardo L, Creasy JM, Petkovska I, Do RRK, Bates DDB, Paroder V, Gonen M, Weiser MR, Simpson AL, Gollub MJ. Radiomics-based prediction of microsatellite instability in colorectal cancer at initial computed tomography evaluation. Abdom Radiol (NY). 2019;44:3755-3763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 125. | Huang X, Cheng Z, Huang Y, Liang C, He L, Ma Z, Chen X, Wu X, Li Y, Liu Z. CT-based Radiomics Signature to Discriminate High-grade From Low-grade Colorectal Adenocarcinoma. Acad Radiol. 2018;25:1285-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 126. | Altini C, Maggialetti N, Branca A, Pisani AR, Rubini D, Sardaro A, Stabile Ianora AA, Rubini G. 18F-FDG PET/CT in peritoneal tumors: a pictorial review. Clin Transl Imaging. 2023;11:141-155. [Cited in This Article: ] |
| 127. | Eresen A, Li Y, Yang J, Shangguan J, Velichko Y, Yaghmai V, Benson AB 3rd, Zhang Z. Preoperative assessment of lymph node metastasis in Colon Cancer patients using machine learning: a pilot study. Cancer Imaging. 2020;20:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Caruso D, Zerunian M, Ciolina M, de Santis D, Rengo M, Soomro MH, Giunta G, Conforto S, Schmid M, Neri E, Laghi A. Haralick's texture features for the prediction of response to therapy in colorectal cancer: a preliminary study. Radiol Med. 2018;123:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 129. | Coppola F, Giannini V, Gabelloni M, Panic J, Defeudis A, Lo Monaco S, Cattabriga A, Cocozza MA, Pastore LV, Polici M, Caruso D, Laghi A, Regge D, Neri E, Golfieri R, Faggioni L. Radiomics and Magnetic Resonance Imaging of Rectal Cancer: From Engineering to Clinical Practice. Diagnostics (Basel). 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 130. | Alvarez-Jimenez C, Antunes JT, Talasila N, Bera K, Brady JT, Gollamudi J, Marderstein E, Kalady MF, Purysko A, Willis JE, Stein S, Friedman K, Paspulati R, Delaney CP, Romero E, Madabhushi A, Viswanath SE. Radiomic Texture and Shape Descriptors of the Rectal Environment on Post-Chemoradiation T2-Weighted MRI are Associated with Pathologic Tumor Stage Regression in Rectal Cancers: A Retrospective, Multi-Institution Study. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 131. | Bordron A, Rio E, Badic B, Miranda O, Pradier O, Hatt M, Visvikis D, Lucia F, Schick U, Bourbonne V. External Validation of a Radiomics Model for the Prediction of Complete Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |